Difference between revisions of "DnaB"
(→References) |
|||
| Line 1: | Line 1: | ||
| − | * '''Description:''' initiation of chromosome replication/ membrane attachment protein <br/><br/> | + | * '''Description:''' initiation of chromosome replication/ membrane attachment protein, part of the [[replisome]] <br/><br/> |
{| align="right" border="1" cellpadding="2" | {| align="right" border="1" cellpadding="2" | ||
| Line 72: | Line 72: | ||
* '''Effectors of protein activity:''' | * '''Effectors of protein activity:''' | ||
| − | * '''Interactions:''' [[DnaB]]-[[PriA]], [[DnaB]]-[[DnaI]], [[DnaB]]-[[DnaC]] [http://www.ncbi.nlm.nih.gov/sites/entrez/11250911 PubMed], [[DnaD]]-[[DnaB]] {{PubMed|15186423,19707554}}, [[DnaB]]-[[DnaX]] [http://www.ncbi.nlm.nih.gov/sites/entrez/14757052 PubMed] | + | * '''Interactions:''' [[DnaB]]-[[PriA]], [[DnaB]]-[[DnaI]], [[DnaB]]-[[DnaC]] [http://www.ncbi.nlm.nih.gov/sites/entrez/11250911 PubMed], [[DnaD]]-[[DnaB]] {{PubMed|15186423,19707554}}, [[DnaB]]-[[DnaX]] [http://www.ncbi.nlm.nih.gov/sites/entrez/14757052 PubMed], part of the [[replisome]]: [[PolC]]-[[HolA]]-[[HolB]]-[[DnaX]]-[[DnaN]]-[[DnaG]]-[[DnaC]]-[[DnaI]]-[[DnaD]]-[[SsbA]]-[[DnaE]]-[[PriA]]-[[DnaB]] {{PubMed|20122408}} |
* '''Localization:''' Membrane-proximal (Spotty) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] forms foci, close to oriC [http://www.ncbi.nlm.nih.gov/sites/entrez/10844689 PubMed] | * '''Localization:''' Membrane-proximal (Spotty) [http://www.ncbi.nlm.nih.gov/sites/entrez/16479537 PubMed] forms foci, close to oriC [http://www.ncbi.nlm.nih.gov/sites/entrez/10844689 PubMed] | ||
| Line 120: | Line 120: | ||
=References= | =References= | ||
| − | <pubmed> 19707554 15686560,12718886,2554322,11250911,15186423,11842108,11585815, 16479537, 19415803, 14757052, 10844689, 12974630 19968790 20071750 11679082 </pubmed> | + | <pubmed> 19707554 15686560,12718886,2554322,11250911,15186423,11842108,11585815, 16479537, 19415803, 14757052, 10844689, 12974630 19968790 20071750 11679082 20122408 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:29, 4 February 2010
- Description: initiation of chromosome replication/ membrane attachment protein, part of the replisome
| Gene name | dnaB |
| Synonyms | |
| Essential | yes PubMed |
| Product | initiation of chromosome replication/ membrane attachment protein |
| Function | DNA replication |
| MW, pI | 54 kDa, 5.278 |
| Gene length, protein length | 1416 bp, 472 aa |
| Immediate neighbours | dnaI, ytcG |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 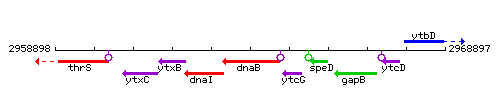
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU28990
Phenotypes of a mutant
essential PubMed
Database entries
- DBTBS entry: no entry
- SubtiList entry: [1]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions: DnaB-PriA, DnaB-DnaI, DnaB-DnaC PubMed, DnaD-DnaB PubMed, DnaB-DnaX PubMed, part of the replisome: PolC-HolA-HolB-DnaX-DnaN-DnaG-DnaC-DnaI-DnaD-SsbA-DnaE-PriA-DnaB PubMed
Database entries
- Structure:
- UniProt: P07908
- KEGG entry: [2]
- E.C. number:
Additional information
Expression and regulation
- Operon:
- Regulation:
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Glenn M Sanders, H Garry Dallmann, Charles S McHenry
Reconstitution of the B. subtilis replisome with 13 proteins including two distinct replicases.
Mol Cell: 2010, 37(2);273-81
[PubMed:20122408]
[WorldCat.org]
[DOI]
(I p)
William H Grainger, Cristina Machón, David J Scott, Panos Soultanas
DnaB proteolysis in vivo regulates oligomerization and its localization at oriC in Bacillus subtilis.
Nucleic Acids Res: 2010, 38(9);2851-64
[PubMed:20071750]
[WorldCat.org]
[DOI]
(I p)
Wiep Klaas Smits, Alexi I Goranov, Alan D Grossman
Ordered association of helicase loader proteins with the Bacillus subtilis origin of replication in vivo.
Mol Microbiol: 2010, 75(2);452-61
[PubMed:19968790]
[WorldCat.org]
[DOI]
(I p)
Megan E Rokop, Alan D Grossman
Intragenic and extragenic suppressors of temperature sensitive mutations in the replication initiation genes dnaD and dnaB of Bacillus subtilis.
PLoS One: 2009, 4(8);e6774
[PubMed:19707554]
[WorldCat.org]
[DOI]
(I e)
Kiran Chintakayala, Cristina Machón, Anna Haroniti, Marilyn A Larson, Steven H Hinrichs, Mark A Griep, Panos Soultanas
Allosteric regulation of the primase (DnaG) activity by the clamp-loader (tau) in vitro.
Mol Microbiol: 2009, 72(2);537-49
[PubMed:19415803]
[WorldCat.org]
[DOI]
(I p)
Jean-Christophe Meile, Ling Juan Wu, S Dusko Ehrlich, Jeff Errington, Philippe Noirot
Systematic localisation of proteins fused to the green fluorescent protein in Bacillus subtilis: identification of new proteins at the DNA replication factory.
Proteomics: 2006, 6(7);2135-46
[PubMed:16479537]
[WorldCat.org]
[DOI]
(P p)
Claude Bruand, Marion Velten, Stephen McGovern, Stéphanie Marsin, Céline Sérèna, S Dusko Ehrlich, Patrice Polard
Functional interplay between the Bacillus subtilis DnaD and DnaB proteins essential for initiation and re-initiation of DNA replication.
Mol Microbiol: 2005, 55(4);1138-50
[PubMed:15686560]
[WorldCat.org]
[DOI]
(P p)
Megan E Rokop, Jennifer M Auchtung, Alan D Grossman
Control of DNA replication initiation by recruitment of an essential initiation protein to the membrane of Bacillus subtilis.
Mol Microbiol: 2004, 52(6);1757-67
[PubMed:15186423]
[WorldCat.org]
[DOI]
(P p)
Anna Haroniti, Christopher Anderson, Zara Doddridge, Laurence Gardiner, Clive J Roberts, Stephanie Allen, Panos Soultanas
The clamp-loader-helicase interaction in Bacillus. Atomic force microscopy reveals the structural organisation of the DnaB-tau complex in Bacillus.
J Mol Biol: 2004, 336(2);381-93
[PubMed:14757052]
[WorldCat.org]
[DOI]
(P p)
A Haroniti, R Till, M C M Smith, P Soultanas
Clamp-loader-helicase interaction in Bacillus. Leucine 381 is critical for pentamerization and helicase binding of the Bacillus tau protein.
Biochemistry: 2003, 42(37);10955-64
[PubMed:12974630]
[WorldCat.org]
[DOI]
(P p)
Marion Velten, Stephen McGovern, Stéphanie Marsin, S Dusko Ehrlich, Philippe Noirot, Patrice Polard
A two-protein strategy for the functional loading of a cellular replicative DNA helicase.
Mol Cell: 2003, 11(4);1009-20
[PubMed:12718886]
[WorldCat.org]
[DOI]
(P p)
P Soultanas
A functional interaction between the putative primosomal protein DnaI and the main replicative DNA helicase DnaB in Bacillus.
Nucleic Acids Res: 2002, 30(4);966-74
[PubMed:11842108]
[WorldCat.org]
[DOI]
(I p)
C Bruand, M Farache, S McGovern, S D Ehrlich, P Polard
DnaB, DnaD and DnaI proteins are components of the Bacillus subtilis replication restart primosome.
Mol Microbiol: 2001, 42(1);245-55
[PubMed:11679082]
[WorldCat.org]
[DOI]
(P p)
S Marsin, S McGovern, S D Ehrlich, C Bruand, P Polard
Early steps of Bacillus subtilis primosome assembly.
J Biol Chem: 2001, 276(49);45818-25
[PubMed:11585815]
[WorldCat.org]
[DOI]
(P p)
M Bárcena, T Ruiz, L E Donate, S E Brown, N E Dixon, M Radermacher, J M Carazo
The DnaB.DnaC complex: a structure based on dimers assembled around an occluded channel.
EMBO J: 2001, 20(6);1462-8
[PubMed:11250911]
[WorldCat.org]
[DOI]
(P p)
Y Imai, N Ogasawara, D Ishigo-Oka, R Kadoya, T Daito, S Moriya
Subcellular localization of Dna-initiation proteins of Bacillus subtilis: evidence that chromosome replication begins at either edge of the nucleoids.
Mol Microbiol: 2000, 36(5);1037-48
[PubMed:10844689]
[WorldCat.org]
[DOI]
(P p)
G Henckes, F Harper, A Levine, F Vannier, S J Séror
Overreplication of the origin region in the dnaB37 mutant of Bacillus subtilis: postinitiation control of chromosomal replication.
Proc Natl Acad Sci U S A: 1989, 86(22);8660-4
[PubMed:2554322]
[WorldCat.org]
[DOI]
(P p)