Difference between revisions of "AddA"
(→References) |
(→References) |
||
| Line 121: | Line 121: | ||
=References= | =References= | ||
| − | <pubmed>,8387145,15610857,7746142, 19129187 15009890 10756102 9781875 </pubmed> | + | <pubmed>,8387145,15610857,7746142, 19129187 15009890 10756102 9781875 16632468 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:59, 14 October 2010
- Description: ATP-dependent deoxyribonuclease (subunit A)
| Gene name | addA |
| Synonyms | recE5 |
| Essential | no |
| Product | ATP-dependent deoxyribonuclease (subunit A)) |
| Function | DNA repair and recombination |
| MW, pI | 140 kDa, 5.127 |
| Gene length, protein length | 3696 bp, 1232 aa |
| Immediate neighbours | addB, sbcD |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 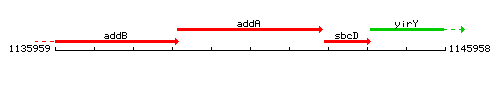
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Locus tag: BSU10630
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity:
- Protein family: uvrD-like helicase C-terminal domain (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Interactions:
- Localization:
Database entries
- Structure:
- UniProt: P23478
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP1106 (addAB, spc), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Joseph T P Yeeles, Richard Cammack, Mark S Dillingham
An iron-sulfur cluster is essential for the binding of broken DNA by AddAB-type helicase-nucleases.
J Biol Chem: 2009, 284(12);7746-55
[PubMed:19129187]
[WorldCat.org]
[DOI]
(P p)
Frédéric Chédin, Naofumi Handa, Mark S Dillingham, Stephen C Kowalczykowski
The AddAB helicase/nuclease forms a stable complex with its cognate chi sequence during translocation.
J Biol Chem: 2006, 281(27);18610-7
[PubMed:16632468]
[WorldCat.org]
[DOI]
(P p)
Alexander Serganov, Yu-Ren Yuan, Olga Pikovskaya, Anna Polonskaia, Lucy Malinina, Anh Tuân Phan, Claudia Hobartner, Ronald Micura, Ronald R Breaker, Dinshaw J Patel
Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs.
Chem Biol: 2004, 11(12);1729-41
[PubMed:15610857]
[WorldCat.org]
[DOI]
(P p)
Etienne Dervyn, Marie-Françoise Noirot-Gros, Peggy Mervelet, Steven McGovern, S Dusko Ehrlich, Patrice Polard, Philippe Noirot
The bacterial condensin/cohesin-like protein complex acts in DNA repair and regulation of gene expression.
Mol Microbiol: 2004, 51(6);1629-40
[PubMed:15009890]
[WorldCat.org]
[DOI]
(P p)
F Chédin, S D Ehrlich, S C Kowalczykowski
The Bacillus subtilis AddAB helicase/nuclease is regulated by its cognate Chi sequence in vitro.
J Mol Biol: 2000, 298(1);7-20
[PubMed:10756102]
[WorldCat.org]
[DOI]
(P p)
F Chédin, P Noirot, V Biaudet, S D Ehrlich
A five-nucleotide sequence protects DNA from exonucleolytic degradation by AddAB, the RecBCD analogue of Bacillus subtilis.
Mol Microbiol: 1998, 29(6);1369-77
[PubMed:9781875]
[WorldCat.org]
[DOI]
(P p)
B J Haijema, L W Hamoen, J Kooistra, G Venema, D van Sinderen
Expression of the ATP-dependent deoxyribonuclease of Bacillus subtilis is under competence-mediated control.
Mol Microbiol: 1995, 15(2);203-11
[PubMed:7746142]
[WorldCat.org]
[DOI]
(P p)
J Kooistra, B J Haijema, G Venema
The Bacillus subtilis addAB genes are fully functional in Escherichia coli.
Mol Microbiol: 1993, 7(6);915-23
[PubMed:8387145]
[WorldCat.org]
[DOI]
(P p)