Difference between revisions of "PtsG"
(→Original publications) |
|||
| Line 175: | Line 175: | ||
<pubmed> 18086213 </pubmed> | <pubmed> 18086213 </pubmed> | ||
==Original publications== | ==Original publications== | ||
| − | <pubmed>10627040 ,12850135 ,18763711 ,11902727 ,9765562 ,9513271 ,1956301 ,10543968 ,17074746 ,15155854 ,14527945 ,1508157 ,2120236 9593197 8418852 1581296 1316146 1733770 1942043 1911744 1906345 20081037 </pubmed> | + | <pubmed>10627040 ,12850135 ,18763711 ,11902727 ,9765562 ,9513271 ,1956301 ,10543968 ,17074746 ,15155854 ,14527945 ,1508157 ,2120236 9593197 8418852 1581296 1316146 1733770 1942043 1911744 1906345 20081037 22846916 </pubmed> |
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 09:03, 1 August 2012
- Description: trigger enzyme: major glucose permease of the PTS, EIICBA(Glc) and control of GlcT activity
| Gene name | ptsG |
| Synonyms | ptsX, crr |
| Essential | no |
| Product | trigger enzyme: glucose-specific enzyme IICBA component of the PTS |
| Function | glucose transport and phosphorylation, control of GlcT activity |
| Interactions involving this protein in SubtInteract: PtsG | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Sugar catabolism | |
| MW, pI | 75,3 kDa, 5.40 |
| Gene length, protein length | 2097 bp, 699 amino acids |
| Immediate neighbours | glcT, ptsH |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 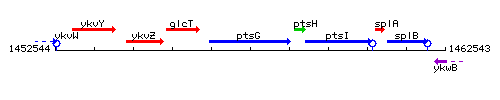
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
phosphotransferase systems, carbon core metabolism, transcription factors and their control, trigger enzyme, membrane proteins, phosphoproteins
This gene is a member of the following regulons
GlcT regulon, stringent response
The gene
Basic information
- Locus tag: BSU13890
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: transport and phosphorylation of glucose, receives a phosphate from HPr at the IIA domain (His-620), the phosphate group is then transferred to the IIB domain (Cys-461) an finally to the incoming glucose. In the absence of glucose, PtsG phosphorylates and thereby inactivates the transcriptional antiterminator GlcT.
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- 11x transmembrane domain (16–36, 89–109, 139–159, 180–200, 233–253, 283–303, 313–333, 338–358, 365–385, 388–408)
- PTS EIIC domain ( 1-424)
- PTS EIIB domain (439–520)
- PTS EIIA domain (568–672)
- Modification: transient phosphorylation (HPr-dependent) on His-620, then internal phosphotransfer from His-620 to Cys-461
- Cofactor(s):
- Effectors of protein activity:
- Localization: membrane protein PubMed
Database entries
- UniProt: P20166
- KEGG entry: [3]
- E.C. number: 2.7.1.69
Additional information
Expression and regulation
- Regulation:
- Regulatory mechanism:
- transcriptional antitermination via the GlcT-dependent RNA switch PubMed
- stringent response: due to presence of guanine at +1 position of the transcript PubMed
- Additional information:
Biological materials
- Mutants: all available in Stülke lab
- Expression vector:
- lacZ fusion:
- pGP34 (pAC5), available in Stülke lab
- pGP66 (pAC7), available in Stülke lab
- pGP606 (mutant terminator, pAC6), available in Stülke lab
- pGP532 (pAC7), available in Stülke lab
- series of promoter deletions are available in pAC5 and pAC6, available in Stülke lab
- series of RAT mutants are available in pAC6, available in Stülke lab
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Fabian M Commichau, Jörg Stülke
Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression.
Mol Microbiol: 2008, 67(4);692-702
[PubMed:18086213]
[WorldCat.org]
[DOI]
(P p)
Original publications