Difference between revisions of "ClpC"
(→Expression and regulation) |
|||
| Line 91: | Line 91: | ||
=Expression and regulation= | =Expression and regulation= | ||
| − | * '''Operon:''' | + | * '''Operon:''' ''[[ctsR]]-[[mcsA]]-[[mcsB]]-[[clpC]]-[[radA]]-[[disA]]'' [http://www.ncbi.nlm.nih.gov/pubmed/8793870 PubMed] |
| − | * '''[[Sigma factor]]:''' [[SigB]] [http://www.ncbi.nlm.nih.gov/pubmed/11544224 | + | * '''[[Sigma factor]]:''' [[SigA]] [http://www.ncbi.nlm.nih.gov/pubmed/8793870 PubMed], [[SigB]] [http://www.ncbi.nlm.nih.gov/pubmed/8793870 PubMed1] [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed2] |
| − | * '''Regulation:''' induced by stress ([[SigB]]) [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed] | + | * '''Regulation:''' induced by stress ([[SigB]]) [http://www.ncbi.nlm.nih.gov/pubmed/11544224 PubMed], induced by heat ([[CtsR]]) [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed] |
| − | * '''Regulatory mechanism:''' | + | * '''Regulatory mechanism:''' [[CtsR]]: transcription repression [http://www.ncbi.nlm.nih.gov/pubmed/9987115 PubMed1], [http://www.ncbi.nlm.nih.gov/pubmed/11179229 PubMed2], [http://www.ncbi.nlm.nih.gov/pubmed/16163393 PubMed3], [http://www.ncbi.nlm.nih.gov/pubmed/17380125 PubMed4] |
* '''Additional information:''' subject to Clp-dependent proteolysis upon glucose starvation [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | * '''Additional information:''' subject to Clp-dependent proteolysis upon glucose starvation [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Abstract&list_uids=+17981983 PubMed] | ||
Revision as of 13:22, 3 May 2009
- Description: ATP-dependent Clp protease, ATPase subunit
| Gene name | clpC |
| Synonyms | mecB |
| Essential | no |
| Product | ATP-dependent Clp protease, ATPase subunit |
| Function | protein degradation
positive regulator of autolysin (LytC and LytD) synthesis |
| MW, pI | 89 kDa, 5.746 |
| Gene length, protein length | 2430 bp, 810 aa |
| Immediate neighbours | mcsB, radA |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 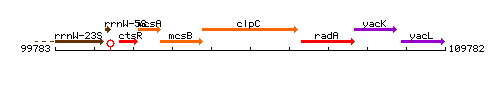
This image was kindly provided by SubtiList
| |
Contents
The gene
Basic information
- Coordinates:
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: ATPase/chaperone
Extended information on the protein
- Kinetic information:
- Domains: AAA-ATPase PFAM
- Modification:
- Cofactor(s):
- Effectors of protein activity:
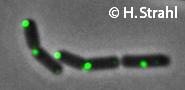
- Localization: cytoplasmic polar clusters, excluded from the nucleoid, induced clustering upon heatshock, colocalization with ClpP Pubmed
Database entries
- Structure:
- Swiss prot entry: P37571
- KEGG entry: [3]
- E.C. number:
Additional information
- subject to Clp-dependent proteolysis upon glucose starvation PubMed
Expression and regulation
- Additional information: subject to Clp-dependent proteolysis upon glucose starvation PubMed
Biological materials
- Mutant: clpC::tet available from the Hamoen] Lab
- Expression vector:
- lacZ fusion:
- GFP fusion: C-terminal GFP fusions (single copy, also as CFP and YFP variants) available from the Hamoen] Lab
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Leendert Hamoen, Newcastle University, UK homepage
Your additional remarks
References
- Petersohn et al. (2001) Global Analysis of the General Stress Response of Bacillus subtilis. J Bacteriol. 183: 5617-5631 PubMed
- Gerth et al. (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190:321-331 PubMed
- Kock H, Gerth U, Hecker M. (2004) MurAA, catalysing the first committed step in peptidoglycan biosynthesis, is a target of Clp-dependent proteolysis in Bacillus subtilis. Mol Microbiol, 51:1087-1102. PubMed
- Kirstein, J., Strahl, H., Molière, N., Hamoen, LW., Turgay K. (2008) Localization of general and regulatory proteolysis in Bacillus subtilis cells. Mol Microbiol. 70:682-94. Pubmed
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed