Difference between revisions of "RsbX"
(→References) |
|||
| Line 149: | Line 149: | ||
=References= | =References= | ||
| − | + | <pubmed>8002610,8682769,9658013,8002609,10671474,9068644, 19923733,11544224, 15466036 8824586 23407164,23320651,21362065 26057679</pubmed> | |
| − | <pubmed>8002610,8682769,9658013,8002609,10671474,9068644, 19923733,11544224, 15466036 8824586 </pubmed> | ||
[[Category:Protein-coding genes]] | [[Category:Protein-coding genes]] | ||
Revision as of 08:12, 10 June 2015
- Description: protein serine phosphatase, feedback PP2C, dephosphorylates RsbS and RsbR
| Gene name | rsbX |
| Synonyms | |
| Essential | no |
| Product | protein serine phosphatase, feedback PP2C |
| Function | control of SigB activity |
| Gene expression levels in SubtiExpress: rsbX | |
| Interactions involving this protein in SubtInteract: RsbX | |
| Metabolic function and regulation of this protein in SubtiPathways: rsbX | |
| MW, pI | 21 kDa, 6.23 |
| Gene length, protein length | 597 bp, 199 aa |
| Immediate neighbours | sigB, ydcF |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 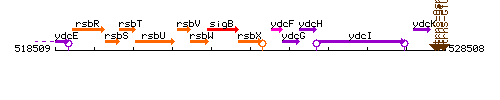
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed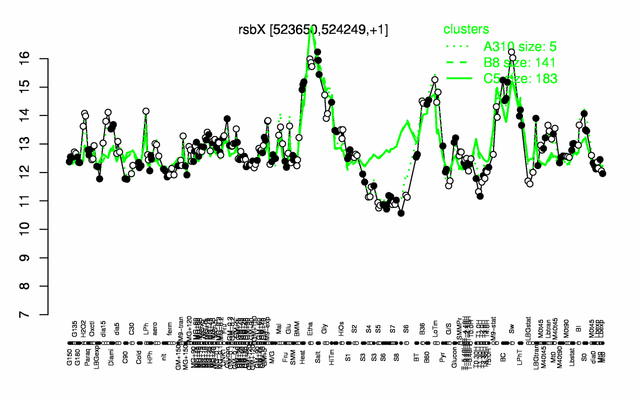
| |
Contents
Categories containing this gene/protein
protein modification, sigma factors and their control, general stress proteins (controlled by SigB)
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU04740
Phenotypes of a mutant
Database entries
- BsubCyc: BSU04740
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Protein family:
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
Database entries
- BsubCyc: BSU04740
- Structure:
- UniProt: P17906
- KEGG entry: [3]
- E.C. number: 3.1.3.3
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
- Bill Haldenwang, San Antonio, USA
- Chet Price, Davis, USA homepage
Your additional remarks
References
Aik Hong Teh, Masatomo Makino, Takeshi Hoshino, Seiki Baba, Nobutaka Shimizu, Masaki Yamamoto, Takashi Kumasaka
Structure of the RsbX phosphatase involved in the general stress response of Bacillus subtilis.
Acta Crystallogr D Biol Crystallogr: 2015, 71(Pt 6);1392-9
[PubMed:26057679]
[WorldCat.org]
[DOI]
(I p)
Jonathan W Young, James C W Locke, Michael B Elowitz
Rate of environmental change determines stress response specificity.
Proc Natl Acad Sci U S A: 2013, 110(10);4140-5
[PubMed:23407164]
[WorldCat.org]
[DOI]
(I p)
Ulf W Liebal, Thomas Millat, Jon Marles-Wright, Richard J Lewis, Olaf Wolkenhauer
Simulations of stressosome activation emphasize allosteric interactions between RsbR and RsbT.
BMC Syst Biol: 2013, 7;3
[PubMed:23320651]
[WorldCat.org]
[DOI]
(I e)
Christine Eymann, Stephan Schulz, Katrin Gronau, Dörte Becher, Michael Hecker, Chester W Price
In vivo phosphorylation patterns of key stressosome proteins define a second feedback loop that limits activation of Bacillus subtilis σB.
Mol Microbiol: 2011, 80(3);798-810
[PubMed:21362065]
[WorldCat.org]
[DOI]
(I p)
Masatoshi Suganuma, Aik Hong Teh, Masatomo Makino, Nobutaka Shimizu, Tomonori Kaneko, Kunio Hirata, Masaki Yamamoto, Takashi Kumasaka
Crystallization and preliminary X-ray analysis of the stress-response PPM phosphatase RsbX from Bacillus subtilis.
Acta Crystallogr Sect F Struct Biol Cryst Commun: 2009, 65(Pt 11);1128-30
[PubMed:19923733]
[WorldCat.org]
[DOI]
(I p)
Chien-Cheng Chen, Michael D Yudkin, Olivier Delumeau
Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis.
J Bacteriol: 2004, 186(20);6830-6
[PubMed:15466036]
[WorldCat.org]
[DOI]
(P p)
A Petersohn, M Brigulla, S Haas, J D Hoheisel, U Völker, M Hecker
Global analysis of the general stress response of Bacillus subtilis.
J Bacteriol: 2001, 183(19);5617-31
[PubMed:11544224]
[WorldCat.org]
[DOI]
(P p)
J M Scott, T Mitchell, W G Haldenwang
Stress triggers a process that limits activation of the Bacillus subtilis stress transcription factor sigma(B).
J Bacteriol: 2000, 182(5);1452-6
[PubMed:10671474]
[WorldCat.org]
[DOI]
(P p)
N Smirnova, J Scott, U Voelker, W G Haldenwang
Isolation and characterization of Bacillus subtilis sigB operon mutations that suppress the loss of the negative regulator RsbX.
J Bacteriol: 1998, 180(14);3671-80
[PubMed:9658013]
[WorldCat.org]
[DOI]
(P p)
U Voelker, T Luo, N Smirnova, W Haldenwang
Stress activation of Bacillus subtilis sigma B can occur in the absence of the sigma B negative regulator RsbX.
J Bacteriol: 1997, 179(6);1980-4
[PubMed:9068644]
[WorldCat.org]
[DOI]
(P p)
X Yang, C M Kang, M S Brody, C W Price
Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor.
Genes Dev: 1996, 10(18);2265-75
[PubMed:8824586]
[WorldCat.org]
[DOI]
(P p)
A Dufour, U Voelker, A Voelker, W G Haldenwang
Relative levels and fractionation properties of Bacillus subtilis σ(B) and its regulators during balanced growth and stress.
J Bacteriol: 1996, 178(13);3701-9 sigma
[PubMed:8682769]
[WorldCat.org]
[DOI]
(P p)
A A Wise, C W Price
Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals.
J Bacteriol: 1995, 177(1);123-33
[PubMed:8002610]
[WorldCat.org]
[DOI]
(P p)
U Voelker, A Dufour, W G Haldenwang
The Bacillus subtilis rsbU gene product is necessary for RsbX-dependent regulation of sigma B.
J Bacteriol: 1995, 177(1);114-22
[PubMed:8002609]
[WorldCat.org]
[DOI]
(P p)