Difference between revisions of "PtsI"
(→Extended information on the protein) |
|||
| Line 66: | Line 66: | ||
**pyrophosphate/phosphate carrier histidine (central Domain) | **pyrophosphate/phosphate carrier histidine (central Domain) | ||
| − | * '''Modification:''' transient autophosphorylation on His-189, in vivo also phosphorylated on Ser-44 or Ser-46 | + | * '''Modification:''' transient autophosphorylation on His-189, in vivo also phosphorylated on Ser-44 or Ser-46 [http://www.ncbi.nlm.nih.gov/pubmed/17218307 PubMed] |
* '''Cofactor(s):''' Magnesium | * '''Cofactor(s):''' Magnesium | ||
Revision as of 18:35, 14 January 2009
- Description: Enzyme I, general (non sugar-specific) component of the PTS. Enzyme I transfers the phosphoryl group from phosphoenolpyruvate (PEP) to the phosphoryl carrier protein (HPr)
| Gene name | ptsI |
| Synonyms | |
| Essential | no |
| Product | phosphotransferase system (PTS) enzyme I |
| Function | PTS-dependent sugar transport |
| MW, pI | 62,9 kDa, 4.59 |
| Gene length, protein length | 1710 bp, 570 amino acids |
| Immediate neighbours | pstH, splA |
| Gene sequence (+200bp) | Protein sequence |
Genetic context 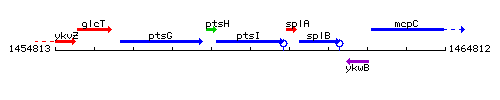
| |
Contents
The gene
Basic information
- Coordinates: 1458959 - 1460668
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: PEP-dependent autophosphorylation on His-189, transfer of the phosphoryl group to HPr (His-15)
- Protein family: PEP-utilizing enzyme family
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- HPr binding site (N-Terminal Domain)
- pyruvate binding site (C-Terminal Domain)
- pyrophosphate/phosphate carrier histidine (central Domain)
- Modification: transient autophosphorylation on His-189, in vivo also phosphorylated on Ser-44 or Ser-46 PubMed
- Cofactor(s): Magnesium
- Effectors of protein activity:
- Interactions:
- Localization: Cytoplasm
Database entries
- Structure:
- Swiss prot entry: [3]
- KEGG entry: [4]
- E.C. number: [5]
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- Antibody:
Labs working on this gene/protein
Josef Deutscher, Paris-Grignon, France
Jörg Stülke, University of Göttingen, Germany Homepage
Your additional remarks
References
- Author1, Author2 & Author3 (year) Title Journal volume: page-page. PubMed
- Pompeo F, Luciano J, Galinier A. (2007) Interaction of GapA with HPr and its homologue, Crh: Novel levels of regulation of a key step of glycolysis in Bacillus subtilis? J Bacteriol. 189(3): 1154-7. PubMed
- Arnaud M, Vary P, Zagorec M, Klier A, Débarbouillé M, Postma P, Rapoport G (1992) Regulation of the sacPA operon of Bacillus subtilis: identification of phosphotransferase system components involved in SacT activity. J Bacteriol 174:3161-3170. PubMed
- Deutscher, J., Kessler, U., Alpert, C. A., and Hengstenberg, W. (1984) Bacterial phosphoenolpyruvate-dependent phosphotransferase system: P-ser-HPr and its possible regulatory function. Biochemistry 23: 4455-4460. DOI:10.1021/bi00314a033
- Deutscher, J., Küster, E., Bergstedt, U., Charrier, V., and Hillen, W. (1995) Protein kinase-dependent HPr/CcpA interaction links glycolytic activity to carbon catabolite repression in Gram-positive bacteria. Mol. Microbiol. 15: 1049-1053. PubMed
- Eisermann, R., Deutscher, J., Gonzy-Tréboul, G., and Hengstenberg, W. (1988) Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. J Biol Chem 263: 17050-17054. PubMed
- Frisby, D., and Zuber, P. 1994. Mutations in pts cause catabolite-resistant sporulation and altered regulation of spo0H in Bacillus subtilis. J. Bacteriol. 176: 2587-2595. PubMed
- Galinier A, Deutscher J, Martin-Verstraete I: (1999) Phosphorylation of either Crh or HPr mediates binding of CcpA to the Bacillus subtilis xyn cre and catabolite repression of the xyn operon. J Mol Biol , 286:307-314. PubMed
- Görke, B., Fraysse, L. & Galinier, A. (2004) Drastic differences in Crh and HPr synthesis levels reflect their different impacts on catabolite repression in Bacillus subtilis. J. Bacteriol. 186, 2992-2995 . PubMed
- Lindner, C., Galinier, A., Hecker, M. & Deutscher, J. (1999) Regulation of the activity of the Bacillus subtilis antiterminator LicT by multiple PEP-dependent, enzyme I- and HPr-catalysed phosphorylation. Mol. Microbiol. 31, 995-1006 . PubMed
- Lindner, C., Hecker, M., Le Coq, D. & Deutscher, J. (2002) Bacillus subtilis mutant LicT antiterminators exhibiting enzyme I- and HPr-independent antitermination affect catabolite repression of the bglPH operon. J. Bacteriol. 184, 4819-4828 . PubMed
- Martin-Verstraete, I., Charrier, V., Stülke, J., Galinier, A., Erni, B., Rapoport, G., & Deutscher, J. (1998) Antagonistic effects of dual PTS catalyzed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol. Microbiol. 28: 293-303. PubMed
- Martin-Verstraete, I., Deutscher, J., and Galinier, A. (1999) Phosphorylation of HPr and Crh by HprK, early steps in the catabolite repression signalling pathway for the Bacillus subtilis levanase operon. J Bacteriol 181: 2966-2969. PubMed
- Reizer, J., Sutrina, S. L., Saier, Jr., M. H., Stewart, G. C., Peterkofsky, A., and Reddy, P. (1989) Mechanistic and physiological consequences of HPr(Ser) phosphorylation on the activities of the phosphoenolpyruvate:sugar phosphotransferase system in Gram-positive bacteria: studies with site-specific mutants of HPr. EMBO J 8: 2111-2120. PubMed
- Schmalisch, M., Bachem, S. & Stülke, J. (2003) Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation: Elucidation of the phosphorylation chain leading to inactivation of GlcT. J. Biol. Chem. 278: 51108-51115. PubMed
- Schumacher, M. A. et al. (2004) Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell 118, 731-741 . PubMed
- Singh, K. D., Halbedel, S., Görke, B. & Stülke, J. (2007) Control of the phosphorylation state of the HPr protein of the phosphotransferase system in Bacillus subtilis: implication of the protein phosphatase PrpC. J. Mol. Microbiol. Biotechnol. 13: 165-171. PubMed
- Singh, K. D., Schmalisch, M. H., Stülke, J. & Görke, B. (2008) Carbon catabolite repression in Bacillus subtilis: A quantitative analysis of repression exerted by different carbon sources. J. Bacteriol. 190: 7275-7284. PubMed
- Stülke, J., Martin-Verstraete, I., Charrier, V., Klier, A., Deutscher, J. & Rapoport, G. (1995) The HPr protein of the phosphotransferase system links induction and catabolite repression of the Bacillus subtilis levanase operon. J. Bacteriol. 177: 6928-6936. PubMed
- Tortosa, P., Aymerich, S., Lindner, C., Saier, M.H., Jr., Reizer, J. and Le Coq, D. (1997) Multiple phosphorylation of SacY, a Bacillus subtilis antiterminator negatively controlled by the phosphotransferase system. J. Biol. Chem. 272, 17230-17237. PubMed