CssS
- Description: two-component sensor kinase, control of cellular responses to protein secretion stress
| Gene name | cssS |
| Synonyms | yvqB |
| Essential | no |
| Product | two-component sensor kinase |
| Function | control of cellular responses to protein secretion stress |
| Metabolic function and regulation of this protein in SubtiPathways: Stress | |
| MW, pI | 51 kDa, 5.795 |
| Gene length, protein length | 1353 bp, 451 aa |
| Immediate neighbours | cssR, yirB |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 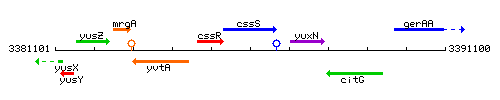
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
protein modification, transcription factors and their control, heat shock proteins, membrane proteins, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33020
Phenotypes of a mutant
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: autophosphorylation, phosphorylation of CssR
- Protein family:
Extended information on the protein
- Kinetic information:
- Domains: two transmembrane segments, C-terminal histidine phosphotransferase domain
- Modification: autophosphorylation on a His residue
- Cofactor(s):
- Effectors of protein activity:
- Localization: cell membrane (according to Swiss-Prot)
Database entries
- Structure:
- UniProt: O32193
- KEGG entry: [3]
- E.C. number:
Additional information
Expression and regulation
- Additional information:
Biological materials
- Mutant:
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Jessica C Zweers, Thomas Wiegert, Jan Maarten van Dijl
Stress-responsive systems set specific limits to the overproduction of membrane proteins in Bacillus subtilis.
Appl Environ Microbiol: 2009, 75(23);7356-64
[PubMed:19820159]
[WorldCat.org]
[DOI]
(I p)
Hanne-Leena Hyyryläinen, Milla Pietiäinen, Tuula Lundén, Anna Ekman, Marika Gardemeister, Sanna Murtomäki-Repo, Haike Antelmann, Michael Hecker, Leena Valmu, Matti Sarvas, Vesa P Kontinen
The density of negative charge in the cell wall influences two-component signal transduction in Bacillus subtilis.
Microbiology (Reading): 2007, 153(Pt 7);2126-2136
[PubMed:17600057]
[WorldCat.org]
[DOI]
(P p)
Elise Darmon, Ronald Dorenbos, Jochen Meens, Roland Freudl, Haike Antelmann, Michael Hecker, Oscar P Kuipers, Sierd Bron, Wim J Quax, Jean-Yves F Dubois, Jan Maarten van Dijl
A disulfide bond-containing alkaline phosphatase triggers a BdbC-dependent secretion stress response in Bacillus subtilis.
Appl Environ Microbiol: 2006, 72(11);6876-85
[PubMed:17088376]
[WorldCat.org]
[DOI]
(P p)
Elise Darmon, David Noone, Anne Masson, Sierd Bron, Oscar P Kuipers, Kevin M Devine, Jan Maarten van Dijl
A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis.
J Bacteriol: 2002, 184(20);5661-71
[PubMed:12270824]
[WorldCat.org]
[DOI]
(P p)
H L Hyyryläinen, A Bolhuis, E Darmon, L Muukkonen, P Koski, M Vitikainen, M Sarvas, Z Prágai, S Bron, J M van Dijl, V P Kontinen
A novel two-component regulatory system in Bacillus subtilis for the survival of severe secretion stress.
Mol Microbiol: 2001, 41(5);1159-72
[PubMed:11555295]
[WorldCat.org]
[DOI]
(P p)
C Fabret, V A Feher, J A Hoch
Two-component signal transduction in Bacillus subtilis: how one organism sees its world.
J Bacteriol: 1999, 181(7);1975-83
[PubMed:10094672]
[WorldCat.org]
[DOI]
(P p)