GltC
- Description: Transcriptional activator of the gltA-gltB operon. Activates expression of the operon in the absence of arginine.
| Gene name | gltC |
| Synonyms | |
| Essential | No |
| Product | transcriptional regulator (LysR family) |
| Function | positive regulation of the glutamate synthase operon (gltAB) |
| Gene expression levels in SubtiExpress: gltC | |
| Interactions involving this protein in SubtInteract: GltC | |
| Metabolic function and regulation of this protein in SubtiPathways: gltC | |
| MW, pI | 33.9 kDa, 5.62 |
| Gene length, protein length | 900 bp, 300 amino acids |
| Immediate neighbours | gltA, proJ |
| Sequences | Protein DNA DNA_with_flanks |
Genetic context 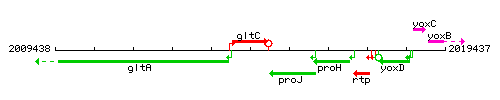
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed
| |
Contents
Categories containing this gene/protein
biosynthesis/ acquisition of amino acids, glutamate metabolism, transcription factors and their control
This gene is a member of the following regulons
The GltC regulon:
The gene
Basic information
- Locus tag: BSU18460
Phenotypes of a mutant
gltC mutants are auxotrophic for glutamate.
Database entries
- BsubCyc: BSU18460
- DBTBS entry: [1]
- SubtiList entry:[2]
Additional information
The protein
Basic information/ Evolution
- Protein family: LysR family PubMed
- Paralogous protein(s): none, but there are 19 members of the LysR family in B. subtilis
Extended information on the protein
- Kinetic information:
- Domains: DNA-binding helix-turn-helix motif: AA 18 ... 37
- Modification:
- Cofactor(s):
- Effectors of protein activity: 2-oxoglutarate stimulates transcription activation, glutamate inhibits transcription activation PubMed
Database entries
- BsubCyc: BSU18460
- Structure:
- UniProt: P20668
- KEGG entry: [3]
Additional information
Expression and regulation
- Regulation: autoregulation by GltC PubMed
- Regulatory mechanism: autorepression PubMed
- Database entries: DBTBS
- Additional information:
- number of protein molecules per cell (minimal medium with glucose and ammonium): 43 PubMed
Biological materials
- Mutant: GP344 (erm), GP738 (spc) (available in Jörg Stülke's lab)
- Expression vector:
- for expression, purification in E. coli with N-terminal His-tag, in pWH844: pGP903, available in Jörg Stülke's lab
- for expression, purification in E. coli with C-terminal Strep-tag, in pET3C: pGP951, available in Jörg Stülke's lab
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Jörg Stülke's lab
- Antibody: available in Jörg Stülke's lab
Labs working on this gene/protein
Linc Sonenshein, Tufts University, Boston, MA, USA Homepage
Jörg Stülke, University of Göttingen, Germany Homepage
Fabian Commichau University of Göttingen, Germany Homepage
Your additional remarks
References
Reviews
Katrin Gunka, Fabian M Commichau
Control of glutamate homeostasis in Bacillus subtilis: a complex interplay between ammonium assimilation, glutamate biosynthesis and degradation.
Mol Microbiol: 2012, 85(2);213-24
[PubMed:22625175]
[WorldCat.org]
[DOI]
(I p)
Sabine Brantl, Andreas Licht
Characterisation of Bacillus subtilis transcriptional regulators involved in metabolic processes.
Curr Protein Pept Sci: 2010, 11(4);274-91
[PubMed:20408793]
[WorldCat.org]
[DOI]
(I p)
Original Publications
Lorena Stannek, Martin J Thiele, Till Ischebeck, Katrin Gunka, Elke Hammer, Uwe Völker, Fabian M Commichau
Evidence for synergistic control of glutamate biosynthesis by glutamate dehydrogenases and glutamate in Bacillus subtilis.
Environ Microbiol: 2015, 17(9);3379-90
[PubMed:25711804]
[WorldCat.org]
[DOI]
(I p)
Katrin Gunka, Joseph A Newman, Fabian M Commichau, Christina Herzberg, Cecilia Rodrigues, Lorraine Hewitt, Richard J Lewis, Jörg Stülke
Functional dissection of a trigger enzyme: mutations of the bacillus subtilis glutamate dehydrogenase RocG that affect differentially its catalytic activity and regulatory properties.
J Mol Biol: 2010, 400(4);815-27
[PubMed:20630473]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Christina Herzberg, Philipp Tripal, Oliver Valerius, Jörg Stülke
A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: the glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC.
Mol Microbiol: 2007, 65(3);642-54
[PubMed:17608797]
[WorldCat.org]
[DOI]
(P p)
Fabian M Commichau, Ingrid Wacker, Jan Schleider, Hans-Matti Blencke, Irene Reif, Philipp Tripal, Jörg Stülke
Characterization of Bacillus subtilis mutants with carbon source-independent glutamate biosynthesis.
J Mol Microbiol Biotechnol: 2007, 12(1-2);106-13
[PubMed:17183217]
[WorldCat.org]
[DOI]
(P p)
Silvia Picossi, Boris R Belitsky, Abraham L Sonenshein
Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC.
J Mol Biol: 2007, 365(5);1298-313
[PubMed:17134717]
[WorldCat.org]
[DOI]
(P p)
Boris R Belitsky, Abraham L Sonenshein
Modulation of activity of Bacillus subtilis regulatory proteins GltC and TnrA by glutamate dehydrogenase.
J Bacteriol: 2004, 186(11);3399-407
[PubMed:15150225]
[WorldCat.org]
[DOI]
(P p)
Ingrid Wacker, Holger Ludwig, Irene Reif, Hans-Matti Blencke, Christian Detsch, Jörg Stülke
The regulatory link between carbon and nitrogen metabolism in Bacillus subtilis: regulation of the gltAB operon by the catabolite control protein CcpA.
Microbiology (Reading): 2003, 149(Pt 10);3001-3009
[PubMed:14523131]
[WorldCat.org]
[DOI]
(P p)
B R Belitsky, A L Sonenshein
Mutations in GltC that increase Bacillus subtilis gltA expression.
J Bacteriol: 1995, 177(19);5696-700
[PubMed:7559360]
[WorldCat.org]
[DOI]
(P p)
D E Bohannon, A L Sonenshein
Positive regulation of glutamate biosynthesis in Bacillus subtilis.
J Bacteriol: 1989, 171(9);4718-27
[PubMed:2548995]
[WorldCat.org]
[DOI]
(P p)