Difference between revisions of "PolY1"
| Line 13: | Line 13: | ||
|- | |- | ||
|style="background:#ABCDEF;" align="center"|'''Function''' || generation of mutations in stationary phase | |style="background:#ABCDEF;" align="center"|'''Function''' || generation of mutations in stationary phase | ||
| + | |- | ||
| + | |colspan="2" style="background:#FAF8CC;" align="center"| '''Gene expression levels in [http://cellpublisher.gobics.de/subtiexpress/ ''Subti''Express]''': [http://cellpublisher.gobics.de/subtiexpress/bsu/BSU23870 polY1] | ||
|- | |- | ||
|colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/carbon_flow.html Central C-metabolism]''' | |colspan="2" style="background:#FAF8CC;" align="center"| '''Metabolic function and regulation of this protein in [[SubtiPathways|''Subti''Pathways]]: <br/>[http://subtiwiki.uni-goettingen.de/pathways/carbon_flow.html Central C-metabolism]''' | ||
Revision as of 11:24, 7 August 2012
- Description: translesion synthesis (TLS-) DNA polymerase Y1, important for stationary phase mutagenesis
| Gene name | polY1 |
| Synonyms | yqjH |
| Essential | no |
| Product | translesion synthesis (TLS-) DNA polymerase Y1 |
| Function | generation of mutations in stationary phase |
| Gene expression levels in SubtiExpress: polY1 | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism | |
| MW, pI | 46 kDa, 9.953 |
| Gene length, protein length | 1242 bp, 414 aa |
| Immediate neighbours | gndA, mifM |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 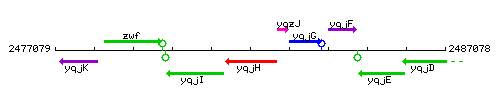
This image was kindly provided by SubtiList
| |
Expression at a glance PubMed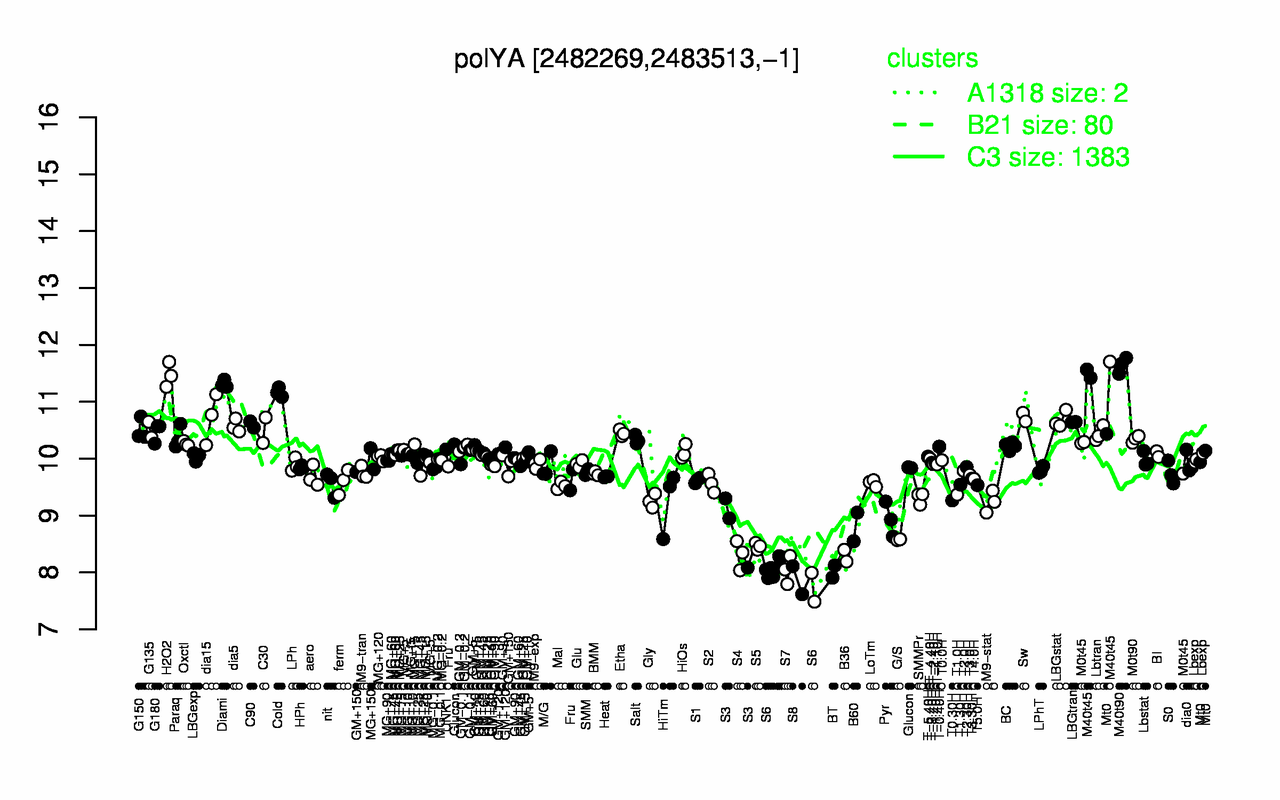
| |
Contents
Categories containing this gene/protein
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU23870
Phenotypes of a mutant
reduced UV-induced mutagenesis PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: Deoxynucleoside triphosphate + DNA(n) = diphosphate + DNA(n+1) (according to Swiss-Prot)
- Protein family: DNA polymerase type-Y family (according to Swiss-Prot)
Extended information on the protein
- Kinetic information:
- Domains:
- Modification:
- Cofactor(s):
- Effectors of protein activity:
- Localization: cytoplasm (homogeneous)
Database entries
- Structure:
- UniProt: P54545
- KEGG entry: [3]
- E.C. number: 2.7.7.7
Additional information
Expression and regulation
- Sigma factor:
- Regulation: constitutively expressed PubMed
- Regulatory mechanism:
- Additional information:
Biological materials
- Mutant: GP1111 (spc), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system:
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Andrea M Rivas-Castillo, Ronald E Yasbin, E Robleto, Wayne L Nicholson, Mario Pedraza-Reyes
Role of the Y-family DNA polymerases YqjH and YqjW in protecting sporulating Bacillus subtilis cells from DNA damage.
Curr Microbiol: 2010, 60(4);263-7
[PubMed:19924481]
[WorldCat.org]
[DOI]
(I p)
Stéphane Duigou, S Dusko Ehrlich, Philippe Noirot, Marie-Françoise Noirot-Gros
DNA polymerase I acts in translesion synthesis mediated by the Y-polymerases in Bacillus subtilis.
Mol Microbiol: 2005, 57(3);678-90
[PubMed:16045613]
[WorldCat.org]
[DOI]
(P p)
Stéphane Duigou, S Dusko Ehrlich, Philippe Noirot, Marie-Françoise Noirot-Gros
Distinctive genetic features exhibited by the Y-family DNA polymerases in Bacillus subtilis.
Mol Microbiol: 2004, 54(2);439-51
[PubMed:15469515]
[WorldCat.org]
[DOI]
(P p)
Huang-Mo Sung, Gabriel Yeamans, Christian A Ross, Ronald E Yasbin
Roles of YqjH and YqjW, homologs of the Escherichia coli UmuC/DinB or Y superfamily of DNA polymerases, in stationary-phase mutagenesis and UV-induced mutagenesis of Bacillus subtilis.
J Bacteriol: 2003, 185(7);2153-60
[PubMed:12644484]
[WorldCat.org]
[DOI]
(P p)