Difference between revisions of "Tpi"
| Line 108: | Line 108: | ||
** ''[[cggR]]-[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} | ** ''[[cggR]]-[[gapA]]-[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} | ||
** ''[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} | ** ''[[pgk]]-[[tpiA]]-[[pgm]]-[[eno]]'' {{PubMed|11489127}} | ||
| + | |||
| + | * '''Expression browser:''' [http://genome.jouy.inra.fr/cgi-bin/seb/viewdetail.py?id=tpiA_3479405_3480166_-1 tpi] {{PubMed|22383849}} | ||
* '''Sigma factor:''' [[SigA]] {{PubMed|11489127}} | * '''Sigma factor:''' [[SigA]] {{PubMed|11489127}} | ||
Revision as of 06:44, 17 April 2012
- Description: triose phosphate isomerase, glycolytic/ gluconeogenic enzyme
| Gene name | tpi |
| Synonyms | tpiA |
| Essential | yes |
| Product | triosephosphate isomerase |
| Function | enzyme in glycolysis/ gluconeogenesis |
| Interactions involving this protein in SubtInteract: Tpi | |
| Metabolic function and regulation of this protein in SubtiPathways: Central C-metabolism, Sugar catabolism | |
| MW, pI | 26,9 kDa, 4.79 |
| Gene length, protein length | 759 bp, 253 amino acids |
| Immediate neighbours | pgm, pgk |
| Get the DNA and protein sequences (Barbe et al., 2009) | |
Genetic context 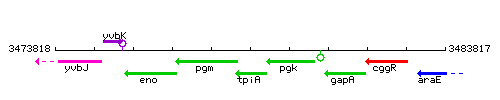
This image was kindly provided by SubtiList
| |
Contents
Categories containing this gene/protein
carbon core metabolism, phosphoproteins
This gene is a member of the following regulons
The gene
Basic information
- Locus tag: BSU33920
Phenotypes of a mutant
- Essential PubMed
Database entries
- DBTBS entry: [1]
- SubtiList entry: [2]
Additional information
- A mutation was found in this gene after evolution under relaxed selection for sporulation PubMed
The protein
Basic information/ Evolution
- Catalyzed reaction/ biological activity: D-glyceraldehyde 3-phosphate = dihydroxyacetone phosphate (according to Swiss-Prot)
- Protein family: triosephosphate isomerase family (according to Swiss-Prot)
- Paralogous protein(s):
Extended information on the protein
- Kinetic information:
- Domains:
- Modification: phosphorylation on Ser-213 PubMed
- Cofactor(s):
- Effectors of protein activity: inhibited by 2-phosphoglycolate (in B. stearothermophilus) PubMed
- Localization:
- cytoplasm PubMed
Database entries
- Structure: 1BTM (complex with 2-phosphoglycolic acid, Geobacillus stearothermophilus), complex with 2-phosphpoglycolic acid, Geobacillus stearothermophilus NCBI
- UniProt: P27876
- KEGG entry: [3]
- E.C. number: 5.3.1.1
Additional information
- extensive information on the structure and enzymatic properties of Tpi can be found at Proteopedia
Expression and regulation
- Regulation:
- Additional information:
Biological materials
- Mutant: GP700 (cat), available in Stülke lab
- Expression vector:
- lacZ fusion:
- GFP fusion:
- two-hybrid system: B. pertussis adenylate cyclase-based bacterial two hybrid system (BACTH), available in Stülke lab
- Antibody:
Labs working on this gene/protein
Your additional remarks
References
Christopher T Brown, Laura K Fishwick, Binna M Chokshi, Marissa A Cuff, Jay M Jackson, Travis Oglesby, Alison T Rioux, Enrique Rodriguez, Gregory S Stupp, Austin H Trupp, James S Woollcombe-Clarke, Tracy N Wright, William J Zaragoza, Jennifer C Drew, Eric W Triplett, Wayne L Nicholson
Whole-genome sequencing and phenotypic analysis of Bacillus subtilis mutants following evolution under conditions of relaxed selection for sporulation.
Appl Environ Microbiol: 2011, 77(19);6867-77
[PubMed:21821766]
[WorldCat.org]
[DOI]
(I p)
Fabian M Commichau, Fabian M Rothe, Christina Herzberg, Eva Wagner, Daniel Hellwig, Martin Lehnik-Habrink, Elke Hammer, Uwe Völker, Jörg Stülke
Novel activities of glycolytic enzymes in Bacillus subtilis: interactions with essential proteins involved in mRNA processing.
Mol Cell Proteomics: 2009, 8(6);1350-60
[PubMed:19193632]
[WorldCat.org]
[DOI]
(I p)
Laurent Jannière, Danielle Canceill, Catherine Suski, Sophie Kanga, Bérengère Dalmais, Roxane Lestini, Anne-Françoise Monnier, Jérôme Chapuis, Alexander Bolotin, Marina Titok, Emmanuelle Le Chatelier, S Dusko Ehrlich
Genetic evidence for a link between glycolysis and DNA replication.
PLoS One: 2007, 2(5);e447
[PubMed:17505547]
[WorldCat.org]
[DOI]
(I e)
Boris Macek, Ivan Mijakovic, Jesper V Olsen, Florian Gnad, Chanchal Kumar, Peter R Jensen, Matthias Mann
The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis.
Mol Cell Proteomics: 2007, 6(4);697-707
[PubMed:17218307]
[WorldCat.org]
[DOI]
(P p)
Hans-Matti Blencke, Georg Homuth, Holger Ludwig, Ulrike Mäder, Michael Hecker, Jörg Stülke
Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways.
Metab Eng: 2003, 5(2);133-49
[PubMed:12850135]
[WorldCat.org]
[DOI]
(P p)
H Ludwig, G Homuth, M Schmalisch, F M Dyka, M Hecker, J Stülke
Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon.
Mol Microbiol: 2001, 41(2);409-22
[PubMed:11489127]
[WorldCat.org]
[DOI]
(P p)
L F Delboni, S C Mande, F Rentier-Delrue, V Mainfroid, S Turley, F M Vellieux, J A Martial, W G Hol
Crystal structure of recombinant triosephosphate isomerase from Bacillus stearothermophilus. An analysis of potential thermostability factors in six isomerases with known three-dimensional structures points to the importance of hydrophobic interactions.
Protein Sci: 1995, 4(12);2594-604
[PubMed:8580851]
[WorldCat.org]
[DOI]
(P p)
M A Leyva-Vazquez, P Setlow
Cloning and nucleotide sequences of the genes encoding triose phosphate isomerase, phosphoglycerate mutase, and enolase from Bacillus subtilis.
J Bacteriol: 1994, 176(13);3903-10
[PubMed:8021172]
[WorldCat.org]
[DOI]
(P p)